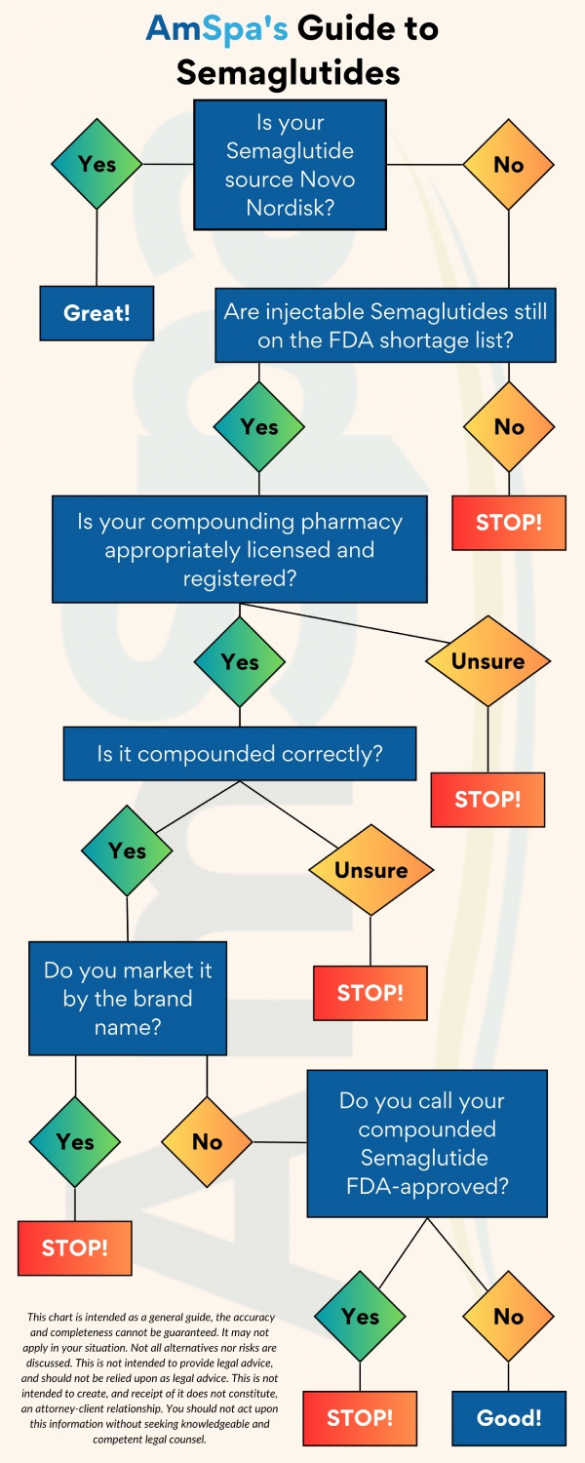
April 24, 2025
U.S. District Court judge denies a motion for a preliminary injunction that would have allowed certain compounding pharmacies to continue producing compounded versions of semaglutide. FDA discretion ends, and deadlines set for 503a and 503b facilities to wind down distribution of compounded tirzepatide.
.
.
.
April 1, 2025
Eli Lilly files lawsuits against two compounding facilities against that were compounding tirzepatide products with added vitamin B3, B12 or glycine, and has accused them of making false suggestions about safety or efficacy.
.
.
.
March 5, 2025
U.S. District Court judge denies a motion for a preliminary injunction that would have allowed certain compounding pharmacies to continue producing compounded versions of tirzepatide. FDA discretion ends, and deadlines set for 503a and 503b facilities to wind down distribution of compounded tirzepatide.
.
.
.
February 21, 2025
Semaglutide shortage is listed as “Resolved” on the FDA Drug Shortages List.
.
.
.
November 1, 2024
All dosages of semaglutide injections are listed as Available on the FDA Drug Shortages List. Status remains listed as “Currently in Shortage.”
.
.
.
August 14, 2024
Tirzepatide shortage is listed as "Resolved" on the FDA Drug Shortages List. One Dosage of semaglutide is still listed as Limited Availability.
.
.
.
August 2, 2024
Tirzepatide is listed as Available on the FDA Drug Shortages List. One Dosage of semaglutide is still listed as Limited Availability.
.
.
.
September 19, 2023
Mounjaro manufacturer Eli Lilly files lawsuits against four compounding pharmacies and six medical spas and wellness centers, for allegedly selling products claiming to contain tirzepatide.
.
.
.
July 6, 2023
Novo Nordisk files lawsuits against four compounding pharmacies in the U.S. for allegedly selling products claiming to contain semaglutide.
.
.
.
June 20, 2023
Novo Nordisk announces intention to sue medical spas, wellness clinics and medical weight loss practices that improperly market and sell products claiming to contain semaglutide.
.
.
.
June 15, 2023
Novo Nordisk and FDA announce counterfeit Ozempic has been found in the U.S.
.
.
.
May 22, 2023
Novo Nordisk pauses key Wegovy promotional efforts to avoid stimulating further demand and ensure product distribution.
.
.
.
May 4, 2023
Novo Nordisk announces it is limiting the supply of Wegovy’s starter doses as a response to growing demand.
.
.
.
April 27, 2023
FDA writes to the National Association Boards of Pharmacy expressing concerns with use of the salt forms of semaglutide (e.g., semaglutide sodium and semaglutide acetate) in compounded products.
.
.
.
September 2022
Ozempic and Wegovy are posted on FDA Drug Shortages list; this allows compounding pharmacies to legally produce similar or even identical products for the duration of the drug’s stay on the list.
.
.
.
March 4, 2022
Novo Nordisk sues six generic-drug makers in Delaware federal court alleging that their proposed cheaper versions of Ozempic infringe on its patents.
.
.
.
March 2022
Wegovy semaglutide injection is shortage-listed with FDA. The shortage halted sales of Wegovy and delayed its launch in the E.U.
.
.
.
December 2021
Novo Nordisk announces that a contract manufacturer in charge of Wegovy syringe filling temporarily stopped deliveries and manufacturing after October 2021 inspection revealed issues with Good Manufacturing Practices.
.
.
.
June 4, 2021
Wegovy is approved by FDA for long-term weight management.
.
.
.
September 20, 2019
Rybelsus (a semaglutide product that is administered orally) is approved by FDA for use in people with type 2 diabetes.
.
.
.
December 5, 2017
Ozempic is approved by FDA for use in people with type 2 diabetes.