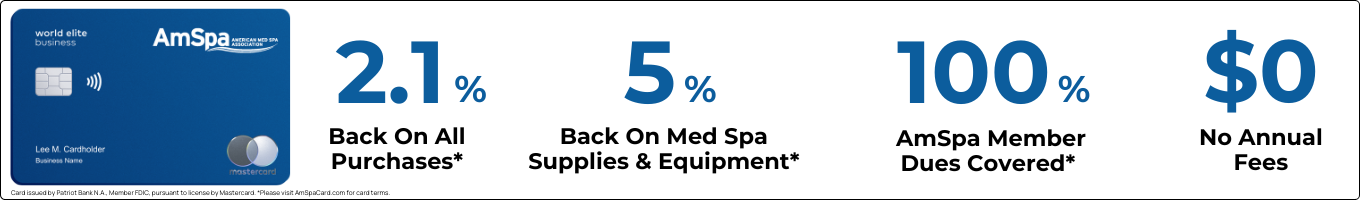
-
Membership
Become a Member
Show your committment to patient safety, legal compliance and community over competition.
-
Training
Join and Save
AmSpa members receive preferred pricing on all AmSpa live and virtual trainings.
-
Blog & News
Latest Blog Posts
View All PostsDon't Miss an Update
Get the latest news and information about safe, legal practice in medical aesthetics directly in your inbox.
-
Resources
Ready to Get Started?
Get access to med spa laws, in-person and online training and more!
- Contact Us
- Become A Member




 Whatever your complexion, it’s important to use products that will help your skin and not damage it. But as you wade through the beauty aisles, the U.S. Food and Drug Administration cautions that you should avoid skin creams, beauty and antiseptic soaps, and lotions that contain mercury.
Whatever your complexion, it’s important to use products that will help your skin and not damage it. But as you wade through the beauty aisles, the U.S. Food and Drug Administration cautions that you should avoid skin creams, beauty and antiseptic soaps, and lotions that contain mercury.